
Titanium (Ti) remains the preferred material for manufacturing dental implants due to its excellent biological and mechanical properties. It has been used in orthopedics to replace hip joints, knee joints, and other joints for decades. Thanks to its high tolerance by the immune system and excellent biocompatibility with very low residues of iron, copper, and nickel when used in its purest form, it has become the standard material for dental implants. It has good biomechanical characteristics, can resist loads under functional conditions, and has an extremely low risk of fracture. However, no metal can be considered completely biocompatible. Titanium has high corrosion resistance, which is attributed to the 10-micron-thick titanium dioxide (TiO₂) layer formed when it contacts with air. Its corrosion rate of less than 0.02 mm per year is far below the acceptable corrosion rate for biomaterials. Nevertheless, titanium implants are located in the oral cavity, which is one of the harshest electrolytic environments in the human body and will be continuously affected by temperature changes, pH fluctuations, and microbial attacks.
Corrosion is affected by pH changes and crevice corrosion. The electrochemical corrosion between titanium and alloys used in dental restorations may damage the TiO₂ layer and cause the release of titanium ions into the surrounding environment. Under normal conditions, due to local and temporal microstructural and environmental changes, titanium ions will be released in the tissues around the implant at a concentration of 100 to 300 ppm. The presence of titanium ions in the tissues around the implant is usually compatible with health, but a concentration exceeding 13 ppm has been proven to be associated with a significant decrease in the survival rate of gingival epithelial cells and has been shown to have a necrotic-inducing effect. In addition, these ions may bind to biomolecules and trigger a Type IV allergic reaction. In the case of infection or mechanical friction, a higher proportion of titanium ions will be found in the body (including the lungs, regional lymph nodes, blood, spleen, or kidneys), and the significance of this remains to be precisely determined.
Reports on titanium allergy can be found in orthopedic literature among patients with failed hip and knee prostheses and those using titanium bone fixation plates. It is believed to account for about 5% of implant failures. The clinical manifestations of intolerance include itching, skin erythema, eczema, oral lichen planus, and granulomatous tissue. There are fewer reports in dental literature on implant loss due to material intolerance. Sicilia et al. described Type IV allergic reactions and their clinical signs in hard and soft tissues in a study report on dental titanium implant allergies based on 1,500 consecutive patients. Patients with obvious allergic symptoms such as perimucosal desquamation, erythema, itching, eczema, dermatitis, or unexplained implant loss, or those with a history of multiple allergies, as well as those exposed to a large amount of titanium due to multiple implant placements or multiple episodes of peri-implantitis and implant loss were studied; 0.6% of implant failures were related to allergic reactions. Yan et al. reported the results based on 217 patients showing positive allergic reactions to at least one metal allergen and found that the prevalence of titanium allergy was 6.3%. De Graaf et al. reported a frequency of 5.7% of titanium allergy in a selected population referred for allergy testing, and this result may not be directly generalized to the general population.
This article reports a case where the implant was removed due to a strong suspicion of titanium allergy and was successfully replaced by a zirconium (Zr) implant.
Case Report
The patient was a 46-year-old female with Hashimoto’s disease (an autoimmune disease that causes gradual destruction of the thyroid gland) and was currently receiving levothyroxine (Euthyrox) treatment and taking daily vitamin supplements. She visited the clinic in June 2016 due to pain after the failure of root canal treatment on the left maxillary first molar (Figure 1). It was decided to extract the affected tooth and perform implant restoration because for the situation of a single tooth missing and adjacent teeth being healthy, dental implants were the best treatment option. The patient’s overall periodontal condition was good, she received regular dental care, and her oral hygiene was good. The left maxillary first molar was extracted; due to the limited bone height under the maxillary sinus, an external maxillary sinus elevation was performed to increase the bone height, and the implant placement was postponed. In July 2016, the maxillary sinus elevation was completed by the lateral window approach. The surgical site was filled with inorganic bovine bone and then allowed to heal for 6 months (Figure 2). Six months later, a cone-beam computed tomography (CBCT) was performed to confirm the healing, and a tapered implant with a diameter of 5 mm and a length of 11.5 mm was placed in Class II bone through a minimally invasive gingival incision with good initial stability. A healing abutment was immediately installed. The patient was treated with antibiotics (amoxicillin/clavulanate potassium, 1 gram twice a day for 5 days) and analgesics (diclofenac 50 mg, taken as needed).
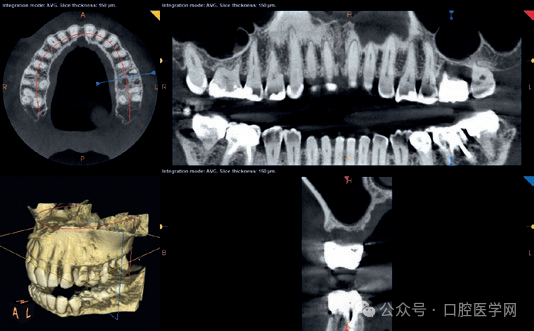
Figure 1
Panoramic X-ray at the first visit. The left maxillary first molar showed severe bone loss due to the failure of root canal treatment. The limited bone height under the maxillary sinus indicated that maxillary sinus floor elevation was required before implant placement.

Figure 2
Local cone-beam computed tomography (CBCT) after lateral window maxillary sinus floor elevation. Please note the change in bone height after the increase and the absence of inflammation in the maxillary sinus mucosa.
A few days after the operation, the patient reported severe pain and a burning sensation in the palate near the implant, and this pain could hardly be controlled by the prescribed analgesics. Clinical examination found a desquamated area about 1 cm wide on the palatal mucosa, similar to a chemical burn, which was initially considered to be a burn caused by hot food. A periodontal dressing was placed in this area to relieve the pain. The patient was seen again one week later, and the inflammation still persisted. Two weeks later, the implant was found to be loose. It was removed with pliers, and all granulation tissues at the surgical site were removed. A CBCT scan was performed, and the results showed inflammatory thickening of the maxillary sinus floor mucosa (Figure 3 and Figure 4). The patient was treated with moxifloxacin 400 mg (1 tablet per day for 1 week). One week later, the pain was completely relieved, and the mucosa was healing normally. This failure that could not be explained from a clinical perspective was suspicious.
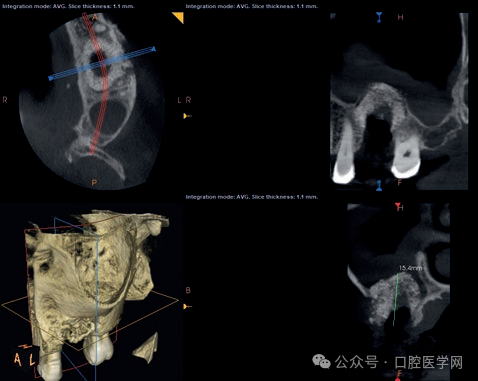
Figure 3
Local cone-beam computed tomography (CBCT) after the removal of the titanium implant. Please note the degree of bone loss at the implant site and the inflammatory thickening of the maxillary sinus mucosa at the left maxillary first molar site.
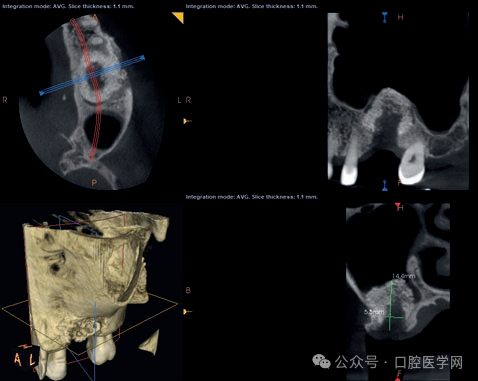
Figure 4
Local cone-beam computed tomography (CBCT) one month after the onset of inflammation. Please note the regression of the maxillary sinus mucosa inflammation and the alveolar defect at the previous implant position, which requires bone grafting.
The patient was referred to a dermatologist (C.I.) for titanium allergy testing. A patch test was performed using the TRUE test, which is an epidermal test for 35 allergens used to diagnose allergic contact dermatitis. In this case, sensitivities to p-phenylenediamine, nickel, cobalt, gold sodium thiosulfate, balsam of Peru, and Disperse Blue were shown. The dermatologist concluded that there was a cross-reaction between titanium and cobalt and nickel, both of which are known titanium co-reactants. However, to confirm titanium allergy, a patch test was performed using a sterile titanium implant cover screw, and the test used a test kit that allowed the allergen to contact the skin. The patch test kit was removed after being exposed on the patient’s back for 48 hours, and readings were taken on the 2nd and 3rd days. According to the reading criteria of the European Society of Contact Dermatology, a positive reaction was rated as +, ++, or +++, which was regarded as an allergy, while a suspicious reaction was not regarded as an allergy. The current patient showed a severe 3+ allergy to the titanium patch test. In addition, a skin prick test of titanium oxide in petrolatum was also performed to provide more evidence of titanium allergy. Compared with the negative saline control and the positive histamine control, this test was also positive (wheal/flare: 4 to 5).
The patient insisted on restoring this tooth. It was proposed to use a zirconium (Zr) implant as an alternative to titanium. The patient agreed to this treatment plan. In April 2017, inorganic bovine bone was added to fill the bone defect caused by the implant failure. The site was allowed to heal for 4 months (Figure 5). In July 2017, a one-piece zirconium implant with a diameter of 4.1 mm and a length of 12 mm, with an abutment 5.5 mm high, was placed without flap elevation and achieved good initial stability. The postoperative process was smooth. The healing of the implant progressed normally, without signs of pain or inflammation. Five months later, a zirconium crown was placed. The 18-month follow-up after the operation confirmed that there were no abnormalities in the hard and soft tissues of the implant (Figure 6a to 6h).

Figure 5
Local cone-beam computed tomography (CBCT) 4 months after bone grafting. Please note the complete bone reconstruction at the implant site and the absence of an inflammatory reaction on adjacent structures.
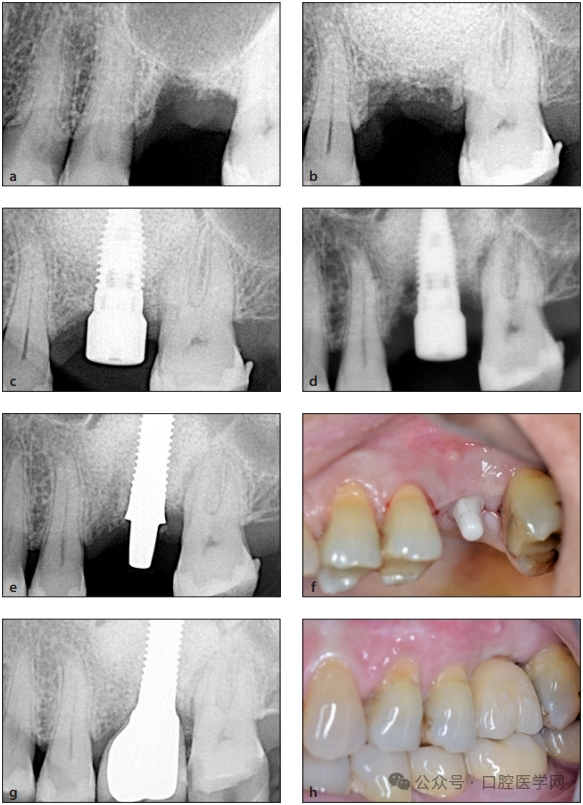
Figure 6
(a) Periapical X-ray before maxillary sinus floor elevation.
(b) Periapical X-ray after maxillary sinus floor elevation.
(c) Periapical X-ray after the placement of the titanium implant and connection of the healing abutment.
(d) Periapical X-ray one week after the implant placement; acute inflammatory reaction led to bone loss around the implant.
(e) Periapical X-ray after the placement of the one-piece zirconium implant.
(f) Clinical image 3 months after the placement of the zirconium implant.
(g) Periapical X-ray when the custom-made zirconium crown was placed.
(h) Final clinical situation 18 months after the operation.
Discussion
This is a rare case of titanium allergy that led to the rapid loss of the implant after a severe Type IV allergic reaction. After all the clinical symptoms of inflammation had subsided and the surgical site had been reconstructed, the titanium implant was successfully replaced by a one-piece zirconium implant. The authors initially explained the palatal gingival desquamation as a burn caused by hot food, and the inflammatory reaction that occurred after the implant placement was initially considered to be caused by infection or trauma. It is important to emphasize that similar postoperative infection cases may be misdiagnosed, and if not properly investigated, the diagnosis of titanium allergy may be overlooked. For cases with unexpected or abnormal reactions to dental implants, titanium allergy testing must be performed to obtain a correct diagnosis.
The inertness and biocompatibility of titanium have recently been questioned by Trindade et al. and Albrektsson et al. According to these authors, osseointegration should not be regarded as a healing process after the insertion of chemically and biologically inert materials, but as a balanced state between immunomodulatory foreign bodies and the immune system. The bone forms around the implant to isolate it from the rest of the body, and as long as this balance is not broken, osseointegration will remain stable. Otherwise, peri-implantitis will start as an immune response and then develop due to microbial contamination.
Titanium allergy has been reported in numerous publications, but the overall prevalence seems to be low. In a study in which 458 patients were tested for titanium allergy using titanium salt panels, 248 were suspected of being allergic to titanium, 163 were suspected of being allergic to other metals rather than titanium, and 47 were in the control group with no suspected titanium allergy; the frequency of titanium sensitivity was 5.7%. In another study evaluating titanium allergy among 1,500 patients, the prevalence was 0.6%. Among a total of 270 patients who visited the allergy department due to suspected metal allergy and received patch tests containing 28 metal and titanium allergens, 80.4% showed positive allergic reactions to at least one metal, and 6.3% were positive for titanium allergens. Sixteen patients had dental implants and reported allergic symptoms after implant placement. Eleven showed positive allergic reactions to one of the tested metals, and 4 were positive for titanium allergens. Five patients were negative for all the tested allergens. None of the patients was positive only for titanium allergens. After one patient’s implant was removed, the allergic reaction completely disappeared. Two patients only removed all metal restorations and superstructures but did not remove the implants, and all allergic symptoms were relieved. One of the four patients with only mild reactions recovered without removing the implants, indicating different individual sensitivity levels to titanium.
Researchers have detected pro-inflammatory cytokines and potential tolerance to titanium in healthy individuals, among whom 14 had no dental implants placed and 6 had dental implants placed without complications. Reactions to titanium dioxide particles and titanium discs were evaluated in terms of lymphocyte transformation test (LTT), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) production, as well as immunomodulation (IL-10). No enhanced T lymphocyte proliferation was observed in any of the subjects. Individuals without implants showed a higher cytokine response than symptom-free implant carriers. Five out of six symptom-free individuals produced IL-10, while none of the 14 control individuals produced IL-10. The authors concluded that the production of IL-1β, IL-6, and TNF-α reflects the normal non-specific immune response to titanium.
Titanium allergy patch tests have been widely used, but there seems to be no clear and reliable testing method, as many tests have been proven unreliable. In a retrospective review, 458 patients received patch tests containing one to five different titanium salts. The detection rate of titanium oxalate hydrate was the highest, while that of titanium dioxide was the lowest. It was finally concluded that medical history and clinical images are still crucial in the diagnosis process. Positive tests in the control group highlight the possibility of false positive results.
In this case, three allergy tests were used. The TRUE test confirmed severe allergies to nickel and cobalt, which have been confirmed to have cross-reactions with titanium. However, since metal allergies such as those to nickel, chromium, and cobalt are not necessarily equivalent to titanium allergy, although patients sensitive to other metals have a higher risk of metal allergy, the epidermal test using a test kit containing titanium and the skin prick test using petrolatum containing titanium oxide showed severe allergic reactions to titanium. The commonly used patch test is less reliable because the epidermal penetration of titanium salts is low. According to Fage et al., given the inconsistencies in the diagnostic tests currently used to determine titanium allergy, the diagnosis of this condition should be mainly based on clinical evaluation. Recently, inflammatory markers such as IL-17 or IL-22 have been used as immunological detection means.
The lymphocyte transformation test (LTT) and the memory lymphocyte immunostimulation assay (MELISA) can be used as alternative diagnostic tools. However, the LTT seems to be more suitable for people who are already sensitive and currently exposed to allergens, rather than those who are already sensitive but currently not exposed to allergens.
After using the MELISA test in patients with negative patch test results after receiving titanium-based implants, positive reactions were induced. However, it is worth noting that among those patients who were positive for metals, 33.9% were negative for titanium allergens. However, after the implants were removed, the clinical symptoms of all patients improved. In addition, these tests produced a large number of false positive results, affecting their validity.
Type I allergy occurs soon after metal exposure and is humoral. Type IV allergy is cell-mediated and occurs several hours to days after exposure to the immunogen. Most allergy reports come from orthopedic literature, but some also involve dental materials. Most of these reports are related to alloy implants containing nickel and cobalt, which may be the cause of implant loosening, but also related to titanium. Du Preez et al. reported a case where a suspected titanium allergy led to a severe tissue reaction at the implant site. Egusa et al. reported a case of a titanium implant complete overdenture accompanied by facial eczema, which completely recovered after the removal of the dental implant. Similarly, a case of contact stomatitis was diagnosed as being related to an abutment coated with titanium nitride, and the symptoms completely disappeared after the abutment was removed and replaced with an uncoated abutment. In another interesting case report, a patient with dental implants developed facial eczema after orthopedic surgery. Although there were no signs of allergy or inflammation at the implant site, the eczema symptoms improved after the removal of the metal prosthesis, and the eczema completely disappeared only after the removal of the dental implant. Zirconium implants have been successfully used to rehabilitate a patient with severe allergies to multiple metals including titanium.
In this case, the diagnosis of titanium allergy was based on the TRUE test, the epidermal test, and the skin prick test, but more importantly, on the adverse clinical symptoms that occurred after implant placement, the regression of symptoms after implant removal, and the good asymptomatic healing after the placement of the zirconium implant. Although the frequency of titanium allergy is considered low, whether due to low prevalence or undiagnosed cases, the importance of developing non-invasive, highly sensitive, and specific tests to confirm titanium allergy still exists in order to identify these rare clinical situations.
References:
1. Steinemann SG. Titanium—the material of choice? Periodontology 2000 1998;17:7–21.
2.Williams DF. Titanium: Epitome of biocompatibility or cause for concern. J Bone Joint Surg Br 1994;76:348–349.
3. Vijayaraghavan V, Sabane AV, Tejas K. Hypersensitivity to titanium: A less explored area of research. J Indian Prosthodont Soc 2012;12:201–207.
4.Chaturvedi T. Allergy related to dental implant and its clinical significance. Clin Cosmet Investig Dent 2013;5:57–61.
5. Suito H, Iwawaki Y, Goto T, Tomotake Y, Ichikawa T. Oral factors affecting titanium elution and corrosion: An in vitro study using simulated body fluid. PLoS One 2013;8:e66052.
6. Torgersen S, Gjerdet NR, Erichsen ES, Bang G. Metal particles and tissue changes adjacent to miniplates. A retrieval study. Acta Odontol Scand 1995;53:65–71.
7. Tawse-Smith A, Ma S, Duncan WJ, Gray A, Reid MR, Rich AM. Implications of wear at the titanium-zirconia implant-abutment interface on the health of peri-implant tissues. Int J Oral Maxillofac Implants 2017;32:599–609.
8. Makihira S, Mine Y, Nikawa H, et al. Titanium ion induces necrosis and sensitivity to lipopolysaccharide in gingival epithelial-like cells. Toxicol In Vitro 2010;24:1905–1910.
9. Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am 2001;83:428–436.
10. Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg 1994;23:450–452.
11. Sicilia A, Cuesta S, Coma G, et al. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin Oral Implants Res 2008;19:823–835.
12. Yan H, Afroz S, Dalanon J, Goto N, Hosoki M, Matsuka Y. Metal allergy patient treated by titanium implant denture: A case report with at least 4-year follow-up. Clin Case Rep 2018;6:1972–1977.
13. de Graaf NPJ, Feilzer AJ, Kleverlaan CJ, Bontkes H, Gibbs S, Rustemeyer T. A retrospective study on titanium sensitivity: Patch test materials and manifestations. Contact Dermatitis 2018;79:85–90.
14. Tawil G, Tawil P, Khairallah A. Sinus floor elevation using the lateral approach and bone window repositioning I: Clinical and radiographic results in 102 consecutively treated patients followed from 1 to 5 years. Int J Oral Maxillofac Implants 2016;31:827–834.
15. Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign body reaction to biomaterials: On mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res 2016;18:192–203.
16. Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction. Clin Implants Dent Relat Res 2014;16:155–165.
17. Thomas P, Iglhaut G, Wollenberg A, Cadosch D, Summer B. Allergy or tolerance: Reduced inflammatory cytokine response and concomitant IL-10 production of lymphocytes and monocytes in symptomfree titanium dental implant patients. Biomed Res Int 2013;2013:539834.
18. Fage SW, Muris J, Jakobsen SS, Thyssen JP. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis 2016;74:323–345.
19. Valentine-Thon E, Müller K, Guzzi G, Kreisel S, Ohnsorge P, Sandkamp M. LTT-MELISA is clinically relevant for detecting and monitoring metal sensitivity. Neuro Endocrinol Lett 2006;27(suppl 1):17–24.
20. Cederbrant K, Hultman P, Marcusson JA, Tibbling L. In vitro lymphocyte proliferation as compared to patch test using gold, palladium and nickel. Int Arch Allergy Immunol 1997;112:212–217.
21. Siddiqi A, Payne AGT, De Silva RK, Duncan WJ. Titanium allergy: Could it affect dental implant integration? Clin Oral Implants Res 2011;22:673–680.
22. du Preez LA, Bütow KW, Swart TJ. Implant failure due to titanium hypersensitivity allergy?—Report of a case. SADJ 2007;62:22–25.
23. Egusa H, Ko N, Shimazu T, Yatani H. Suspected association of an allergic reaction with titanium dental implants: A clinical report. J Prosthet Dent 2008;100:344–347.
24. Lim HP, Lee KM, Koh YI, Park SW. Allergic contact stomatitis caused by a titanium nitride-coated implant abutment: A clinical report. J Prosthet Dent 2012;108:209–213.
25. Hosoki M, Nishigawa K, Miyamoto Y, Ohe G, Matsuka Y. Allergic contact dermatitis caused by titanium screws and dental implants. J Prosthodont Res 2016;60:213–219.
26. Oliva X, Oliva J, Oliva JD. Full-mouth oral rehabilitation in a titanium allergy patient using zirconium oxide dental implants and zirconium oxide restorations. A case report from an ongoing clinical study. Eur J Esthet Dent 2010;5:190–203.




Leave a Reply