
Non-Surgical Treatment of Inferior Alveolar Nerve Injury
Currently, the best time for repair is immediately after damage and during the acute phase. Long-term anti-inflammatory observation is not beneficial for patients, and early referral is necessary to maximize the potential for nerve recovery. Non-surgical treatment, which is less sensitive to technique, is crucial after damage and includes immediate intraoperative handling, postoperative medication and cryotherapy, sensory retraining, and various physical therapies. These are applicable to inferior alveolar nerve injuries, lingual nerve injuries, and mental nerve injuries. Nerve injury healing involves cellular repair. The number of cells does not increase after injury, but when conditions are suitable, axons cross the injury gap to reconnect with the distal nerve and re-establish functional contact. Some studies indicate that nerve injuries with transient sensory disturbances often recover spontaneously, but sensory dysfunction caused by implant surgery usually worsens over time, necessitating active treatment. There is no consensus on the best strategies for implant-related nerve injuries, but several scholars have recommended very similar strategies covering both early and late stages of injury, as follows:
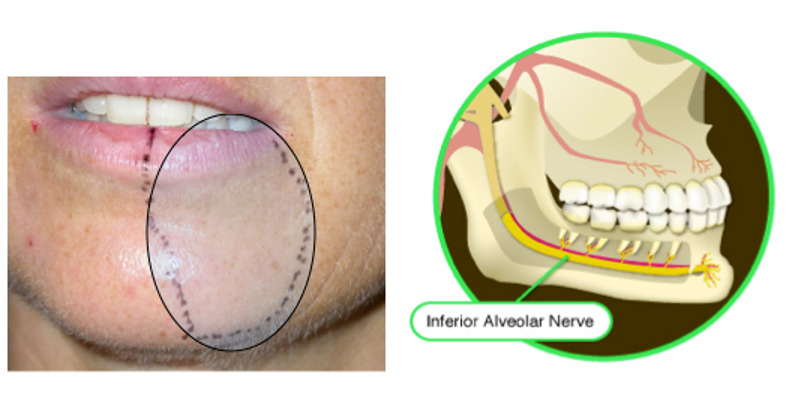
If intraoperative nerve compression injury is suspected or transection injury is observed, imaging examination is recommended postoperatively. If nerve transection is confirmed, immediate referral to a neurology specialist is advised. If there is no obvious nerve transection but the inferior alveolar nerve canal is invaded by the implant, the implant should be removed, and a local injection of 4-8 g/L dexamethasone 1-2 mL should be administered. 24 mg of dexamethasone should be taken orally, 2 tablets in the morning for 3 days, then reduced to 1 tablet in the morning. Cryotherapy is recommended for 1 week. If the implant does not contact the inferior alveolar nerve canal in three dimensions, there is no need to reposition or remove the implant. If nerve sensory abnormalities persist 1 week post-surgery, sensory testing evaluation is needed, and a large dose of non-steroidal anti-inflammatory drugs (NSAIDs) should be administered. If painful dysesthesia or complete sensory loss occurs, timely referral to a neurology specialist is suggested. If nerve sensory abnormalities persist 12 weeks (8 weeks post-surgery), follow-up nerve sensory testing should be conducted every two weeks, and long-term medication should be given. If there is no improvement in pain or sensation, referral to a neurology specialist for surgical consideration is advised.
1. **Immediate Intraoperative Handling**
If sudden wall penetration, an electric shock sensation, or inferior alveolar arteriovenous bleeding occurs during surgery, it is recommended not to place the implant and to wait 2-3 days to confirm no nerve damage before implantation. If mild trauma such as pulling or compression is observed intraoperatively, a local injection of dexamethasone (4 g/L, 1-2 mL) is recommended, acting for 1-2 minutes. If symptoms arise from bone compression of the nerve canal after implantation, it is recommended to retract the implant a few turns or use a shorter implant. If the implant invades the nerve canal, it should be removed as soon as possible. Early removal of the implant (within 24-48 hours, within 30 hours, within 36 hours) can minimize neuropathy. Some scholars mention that the success rate of alleviating or reversing neuropathy symptoms significantly declines if the implant is removed after 36 hours. Additionally, other scholars believe that once initial healing occurs after implant removal, re-implantation is possible.
2. **Postoperative Medication and Cryotherapy**
Clinicians are advised to actively follow up within 6-24 hours after implant surgery. Some scholars suggest that local injection of phentolamine mesilate (PM, 0.4 mg/1.7 mL) can reduce the anesthetic dissipation time from 3-5 hours to about 1 hour, facilitating rapid diagnosis, accurate assessment, and timely treatment. High doses of corticosteroids within one week of injury can minimize neuropathy and prevent neuroma formation. A stepped reduction of corticosteroids for 5-7 days post-injury is recommended, with 8-12 mg of dexamethasone having stronger anti-inflammatory effects than other corticosteroids. The specific regimen: 4 mg dexamethasone, 2 tablets in the morning daily for 3 days, then 1 tablet in the morning. Alternatively, reduce gradually: 8 mg daily on days 1-2, 6 mg daily on days 3-4, 4 mg daily on days 5-6, and 2 mg on day 7. Many surgeons routinely use preoperative local steroid injections, but its benefit for anti-inflammatory medication post-nerve injury is inconclusive. Some scholars believe that due to the very low drug penetration rate of corticosteroids into the inferior alveolar nerve canal, the therapeutic effect on inferior alveolar nerve injury may not be significant, but relevant clinical trials are lacking. NSAIDs are very effective as an adjunct to corticosteroids in the initial 1-3 weeks of injury. Their combined use can minimize inflammation and damage to the nerves. If the injury is severe, it is recommended to start high-dose NSAIDs (ibuprofen 600-800 mg three times a day for 3 weeks) alongside steroid treatment. If no gastrointestinal reaction occurs, NSAIDs treatment can be extended for another 3 weeks based on examination results. Furthermore, studies indicate that preoperative use of corticosteroids and NSAIDs can effectively prevent persistent nerve injury. If nerve sensory symptoms persist after implant removal, immediate use of high-dose ibuprofen and prednisolone can maximize the reduction of inflammation and nerve damage. Some scholars recommend doses of ibuprofen 800 mg three times a day, plus prednisolone 50 mg once a day, or 1 mg/(kg·d) (up to 80 mg) for the first week, then reduce by 10 mg daily for 5 days. Caution is needed as oral dexamethasone should be avoided in patients with heart disease, hypertension, and/or kidney disease, as it may cause hypertensive crises. Long-term combined use of NSAIDs and corticosteroids can cause severe complications such as gastrointestinal ulcers. Even for short-term use, cautious administration and medication history documentation are necessary. It should be noted that patients with chronic pain often have a history of misuse or abuse of medication, and long-term medication should be avoided in treatment design. Psychological counseling, surgery, and other treatment options should be considered promptly. Mecobalamin or other B vitamins, vitamin A, and multivitamins as neurotrophic drugs have also been positively applied. Antibiotics can be used to prevent infection-related perineural edema.

3. **Sensory Retraining**
Sensory retraining is a cognitive-behavioral therapy technique that helps patients adapt to sensory changes effectively and rebuild sensation with visual participation, achieving some functional recovery and reducing pain. Training frequency is more important than duration, typically 3-4 times a day, each session lasting a few minutes, continuing for more than 12 months. The early stage of retraining aims to relearn new tactile sensations, their actual intensity, and location, while the later stage focuses on adjusting the tactile movement direction perceived by the patient. This training only improves the patient’s sensory adaptation ability and does not affect nerve regeneration or absolute tactile thresholds. Generally, the sensory level after one year is the final sensory level a patient can achieve. Animal experiments and imaging studies support the improvement in sensory resolution after training.
Finally, while implementing the above strategies, follow-up with patients on the 7th, 14th, and 21st days and at 1, 2, and 3 months is recommended, and continuous psychological support should be provided.




Leave a Reply