In recent years, the use of metal restorations in the field of dentistry has gradually decreased, as the aesthetic requirements for restorations have increased. In contrast, zirconia all-ceramic restorations offer high aesthetics and exhibit good mechanical properties such as hardness, strength, corrosion resistance, and fatigue resistance, as well as excellent biocompatibility. However, due to the differences between zirconia and glass ceramics, achieving ideal bonding results for zirconia restorations remains challenging for clinicians.
Surface Treatment of Zirconia Ceramics:
In recent years, researchers have tested different surface treatments, primers, adhesives, and various types of resin cements for zirconia ceramics. However, a standardized bonding process for zirconia all-ceramic crowns has not been established yet. Based on the current published research, three important steps are considered essential for successful bonding of all-ceramic restorations: surface treatment of the restoration, adhesive treatment of the prepared tooth structure, and the proper selection of adhesive agent depending on the type of restoration. This article analyzes the existing research progress and summarizes the surface treatment methods and adhesive materials for bonding zirconia restorations, providing a theoretical basis for improving the bonding performance of zirconia ceramics.
Standard Operating Procedure for Zirconia Bonding:
1.1 Bonding Principles of Zirconia Zirconia primers contain 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP). One end of 10-MDP has a C=C group, while the other end has a phosphoric acid group (-OP(OH)2), which exhibits hydrophobic and hydrophilic properties, respectively. The hydrophobic end can undergo polymerization reaction with the C=C group in the resin matrix before polymerization, completing the curing process and co-polymerizing with the resin monomers. The hydrophilic phosphoric acid group can form an ionic bond with the calcium ions in hydroxyapatite of the dentin tubules, creating a hydrophobic nanolayer that enhances the durability of dentin bonding and prevents the decrease in bond strength caused by hydrolysis. The phosphoric acid group can also form a covalent bond (P-O-Zr) with the O-Zr-O molecules on the zirconia surface, achieving stable chemical bonding.
1.2 Standardized Bonding Procedure for Zirconia:
The preparation of the tooth surface involves cleaning the tooth to remove contaminants such as saliva and blood. The tooth surface is cleaned with pumice and rubber cups, followed by thorough rinsing and drying. During the insertion and bonding process of the restoration, the area around the tooth must be kept dry. For patients with excessive saliva or lower jaw restorations, the use of rubber dam isolation is recommended. The treatment of the restoration surface includes intraoral try-in, adjustments, and polishing. The restoration is cleaned, contaminants are removed using ethanol, and the surface is sandblasted. Special restoration cleaning agents are used to clean the surface, followed by thorough drying. When bonding the restoration with adhesive, the surface of the restoration can be treated with the primer provided by the adhesive system. According to the instructions for use of the adhesive, it may be necessary to separately acid-etch the tooth bonding surface, with strict isolation of saliva to prevent contamination and ensure proper bonding. After bonding, the occlusal contact and the contact between the restoration and adjacent teeth are checked, and any remaining adhesive should be carefully removed. Finally, the restoration margins are polished using rubber cups and pumice.
Surface Treatment of Zirconia Ceramics:
Due to the strong stability of zirconia ceramics, it is difficult to achieve a chemical bond with adhesive agents. Hydrofluoric acid etching and the use of silane coupling agents have not significantly improved the bonding strength. Therefore, current research focuses on two aspects of surface treatment for zirconia ceramics: mechanical surface roughening to increase the bonding area and micro-mechanical interlocking and the preparation of silica-coated surfaces and the use of acidic functional monomer adhesives to enhance bonding strength and reduce the chance of restoration debonding.

2.1 Physical Methods:
2.1.1 Sandblasting:
Sandblasting is one of the most common methods, which uses high-speed aluminum oxide particles to impact and release energy. Zirconia material is eroded, resulting in a rough and clean surface. However, sandblasting may also cause surface damage, defects, and the formation of cracks, which can affect its mechanical properties. It is recommended to use appropriate parameters related to pressure, distance from the source, and particle size for sandblasting [2].
2.1.2 Laser:
Laser treatment is a mechanical conditioning technique for bonding zirconia. Commonly used dental lasers include Er:YAG laser, Nd:YAG laser, semiconductor laser, and diode laser. The goal is to increase surface roughness for better micromechanical interlocking with the resin. Overheating zirconia can lead to cracks, residual stresses, and monoclinic phase transformation. Studies on ultrashort pulse lasers (Yb:YAG) have shown that the laser emits pulses of approximately 6 ps, with a power of 9 W. The short pulse duration allows the selective removal of a small amount of laser energy absorbed by the material, resulting in minimal mechanical and thermal damage to the remaining parts of the sample [4].
2.1.3 Electrical Discharge Machining:
Electrical discharge machining is an unconventional method that corrodes the material through electric pulses in a dielectric medium [5].

2.1.4 Selective Infiltration Etching (SIE) Technique:
The principle of this technique is to coat the ceramic with a silicon-based material that has a similar coefficient of thermal expansion to zirconia. Heating zirconia at a certain temperature for a certain period causes a change in the grain structure, resulting in grain growth or the formation of cubic grains [6]. This technique can control grain changes by adjusting temperature and heating time. Selective infiltration etching introduces a molten glass infiltrant into the zirconia grains by surface tension, promoting grain boundary diffusion. After acid etching during bonding, the infiltrant surrounding the grains is removed, leaving a three-dimensional network structure formed by the gaps between the grains. This increases surface roughness. Resin cements can penetrate these networks and form strong mechanical bonds [7]. Selective infiltration etching only treats the surface in contact with the infiltrant, selectively creating gaps in a controlled area without causing cracks or scratches in zirconia, highlighting its advantages over traditional sandblasting. Numerous studies have shown that selective infiltration etching, compared to traditional treatment methods (alumina sandblasting), can significantly improve the bonding strength with resin cements [8].
2.2 Chemical Methods:
2.2.1 Organic Phosphate Monomers and Silane Coupling Agents:
The most widely used phosphate ester monomer is 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), which is commonly added to primers or adhesives to improve the bonding performance between resin cements and zirconia [9,10]. 10-MDP has a phosphoric acid group at one end, which reacts with zirconia and forms a P-O-Zr bond. The other end of the molecule is occupied by an ethylene-based moiety, which can co-polymerize with resin monomers. Silane coupling agents contain a silane group that hydrolyzes in solvents to form silanol groups, which can react with hydroxylated zirconia surfaces through condensation reactions. Silane can also enhance the wettability of zirconia surfaces, thereby increasing the bond strength [11]. Frictional chemical silica coating technology can deposit a silica layer on the zirconia surface, improving the bonding effect. Some universal adhesives contain both 10-MDP and silane molecules to achieve the best bonding effect. However, the mixture of multiple components may lead to competition for the zirconia surface, preventing the functional monomer from exerting its optimal effect.
2.2.2 Silicone Coating for Surface Activation:
Zirconia is a polycrystalline ceramic that is generally resistant to acid etching. To promote adhesion, some authors have investigated the possibility of applying silica-rich glass layers to the zirconia surface to treat it like glass ceramics. Etching with hydrofluoric acid and using silane as a coupling agent have been studied. The silane molecule has two different functional groups: the -SiOH group can bond with hydroxyl groups on the silica coating surface, forming siloxane bonds, and the other functional groups can bond with the methacrylate groups in resin adhesives [12]. However, research has shown that this glass layer may be fragile and can lead to surface defects and crack propagation.
Silicatization of the zirconia surface can also be achieved through a “thermochemical” technique. For example, the Silano-Pen system consists of a lighter with a solution containing butane and silane. When the butane is burned, the silane compound decomposes into SiOx-C fragments that adhere to the zirconia, resulting in silicatization [13]. This method is not efficient enough to promote stable and enduring chemical bonds with the composite materials.

2.2.3 Polydopamine Composite Coating:
It has been found that a polydopamine coating can enhance the integration of soft tissues around zirconia implants and reduce bacterial activity [14]. Therefore, polydopamine coating can increase the surface energy of zirconia, thus enhancing the bond strength. Polydopamine composite coating mainly involves two substances: polydopamine and tetrathiol polyol acid ester. The phenolic groups in dopamine molecules play a chelating role, allowing organic compounds to adhere to the surface of metals or other materials. The catecholamine functional groups in polydopamine can introduce active groups such as carboxyl and amino groups into the zirconia surface, increasing the surface energy. They can also react with the functional monomers in resin adhesives to form chemical bonds. Additionally, acrylate monomers in resin adhesives undergo inhibition under aerobic conditions, which can affect bond strength to some extent. The non-reactive groups in tetrathiol polyol acid ester can react with acrylate materials, further enhancing chemical bonding. Research has shown that when polydopamine composite coatings are used in combination with adhesive agents containing functional monomers, the bond strength can be further improved [15].
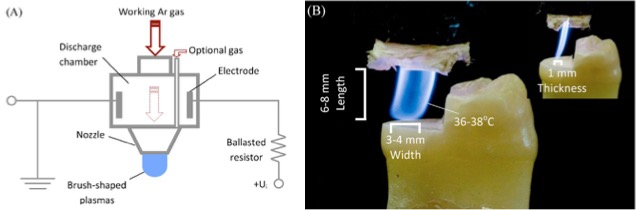
2.3 Plasma Surface Modification:
Plasma surface modification involves using high-active particles to break larger polymer chains into smaller particles, breaking chemical bonds such as C-H and C-C, and dissolving debris through chemical reduction to achieve a clean effect without changing the surface morphology. This method can be used to increase the bond strength of ceramics [16]. Studies have shown that plasma treatment samples have lower bond strength compared to the isopropanol ultrasound cleaning group [17]. However, Güers et al. proposed a combined approach of 99% ultrasound isopropanol cleaning and plasma treatment (argon or air) to achieve a combination of chemical and mechanical cleaning effects, which is more effective in cleaning zirconia contaminated by saliva [18].
Conclusion:
Due to the strong stability of zirconia, it is difficult to achieve a chemical bond with adhesive agents. Hydrofluoric acid etching and the use of silane coupling agents have not significantly improved bonding strength. Therefore, researchers have focused on studying the surface treatment of zirconia ceramics, and the combination of physical sandblasting and chemical reactions is currently the most common method. However, each method has its advantages and limitations, and further improvement of existing materials and techniques is still required to standardize bonding procedures and broaden the clinical application of zirconia ceramics.
References:
[1] Iketani Y, Kobayashi M, Niizuma Y, et al.Effect of various resin cements and immediate dentin sealing on tooth fracture resistance of zirconia inlay restorations[J].American Journal of Dentistry,2021, 34 (4): 179-185.
[2] Scaminaci Russo D, Cinelli F, Sarti C, Giachetti L.Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials[J].Dentistry journal,2019, 7 (3): 74.
[3] Alshammary F, Karobari M I, Assiry A A, et al.Effect of Nd: YAG, Er, Cr: YSGG laser irradiation, and adjunctive photodynamic therapy on push-out bond strength of zirconia posts to radicular dentin[J].BioMed Research International,2021, 2021: 1-7.
[4] Kern M, Passia N, Sasse M, Yazigi C.Ten-year outcome of zirconia ceramic cantilever resin-bonded fixed dental prostheses and the influence of the reasons for missing incisors[J].Journal of dentistry,2017, 65: 51-55.
[5] Rona N, Yenisey M, Kucukturk G, et al.Effect of electrical discharge machining on dental Y-TZP ceramic-resin bonding[J].Journal of prosthodontic research,2017, 61 (2): 158-167.
[6] Wattanasirmkit K, Charasseangpaisarn T.Effect of different cleansing agents and adhesive resins on bond strength of contaminated zirconia[J].Journal of Prosthodontic Research,2019, 63 (3): 271-276.
[7] Çakırbay Tanış M, Akay C, Şen M.Effect of selective infiltration etching on the bond strength between zirconia and resin luting agents[J].Journal of Esthetic and Restorative Dentistry,2019, 31 (3): 257-262.
[8] Salem R, Naggar G, Aboushelib M, Selim D.Microtensile bond strength of resin-bonded hightranslucency zirconia using different surface treatments[J].J Adhes Dent,2016, 18 (3): 191-6.
[9] Yang L, Chen B, Xie H, et al.Durability of resin bonding to zirconia using products containing 10-methacryloyloxydecyl dihydrogen phosphate[J].J Adhes Dent,2018, 20 (4): 279-87.
[10] Li X, Liang S, Inokoshi M, et al. Different surface treatments and adhesive monomers for zirconia-resin bonds: A systematic review and network meta-analysis[J]. Japanese Dental Science Review, 2024, 60: 175-189.
[11]Lima R B W, Barreto S C, Alfrisany N M, et al.Effect of silane and MDP-based primers on physico-chemical properties of zirconia and its bond strength to resin cement[J].Dental materials,2019, 35 (11): 1557-1567.
[12] Albuquerque P, Duarte M, Moreno M B P, et al.Physicochemical properties and microshear bond strength of experimental self-adhesive resin cements to dentin or yttria-stabilized tetragonal zirconia polycrystal[J].J Adhes Dent,2019, 21 (2): 133-41.
[13] Sagen M A, Kvam K, Ruyter E I, Rønold H J.Debonding mechanism of zirconia and lithium disilicate resin cemented to dentin[J].Acta biomaterialia odontologica Scandinavica,2019, 5 (1): 22-29.
[14]江楠, 王楠, 张懿范, et al.氧化锆粘接处理剂的临床应用与前景[J].中国组织工程研究,2021, 25 (10): 1635.
[15] Farhan F A, Sulaiman E, Kutty M G.Effect of new zirconia surface coatings on the surface properties and bonding strength of veneering zirconia substrate[J].Surface and coatings technology,2018, 333: 247-258.
[16] Bitencourt S B, Dos Santos D M, Da Silva E V, et al.Characterisation of a new plasma-enhanced film to improve shear bond strength between zirconia and veneering ceramic[J].Materials Science and Engineering: C,2018, 92: 196-205.
[17] Chen M, Zhang Y, Driver M S, et al.Surface modification of several dental substrates by non-thermal, atmospheric plasma brush[J].Dental Materials,2013, 29 (8): 871-880.
[18] Güers P, Wille S, Strunskus T, et al.Durability of resin bonding to zirconia ceramic after contamination and the use of various cleaning methods[J].Dental Materials,2019, 35 (10): 1388-1396.




Leave a Reply