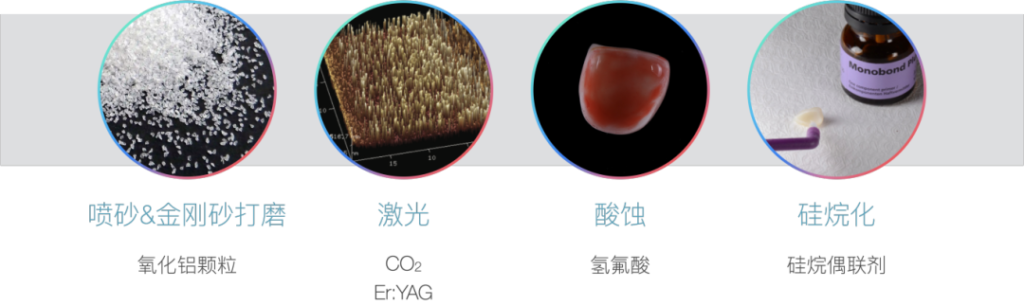
The bonding technology of ceramics, as one of the commonly used techniques in oral restoration clinical practice, has developed relatively maturely at present. One of the prerequisites for ensuring the long – term stability of bonding is the pretreatment of the ceramic surface. Currently, the all – ceramic restoration materials used clinically include silicate – based, zirconia – based, and alumina – based materials.
Regarding lithium disilicate, traditional feldspathic ceramics have problems such as low compressive and flexural strength and poor fracture toughness. Since the 1980s, all – ceramic lithium disilicate with better aesthetic simulation has been widely used in clinical practice and has become one of the mainstream restoration materials. As a silicate – based material, the ability to be etched by acid is the most significant feature that differentiates lithium disilicate from high – strength alumina and zirconia ceramics. The realization of its good bonding effect depends on micro – mechanical physical locking and chemical bonding of hydroxyl groups.
I. Physical Modification
To obtain a micro – mechanically interlocked structure, the ceramic surface needs to be roughened to obtain surface activity. The main current surface treatment methods include alumina sandblasting and emery instrument grinding. Through sandblasting, the crystal structure of lithium disilicate disappears, and irregular micro – mechanical retention forms such as depressions and cracks are formed, which is conducive to the embedding of resin and the improvement of bonding strength.
For sandblasting – type surface roughening, we need to note that over – sandblasting should be avoided. Experimental results show that over – sandblasting will lead to the generation of cracks, fragments, and even volume loss, and reduce the fit, sealing, and strength of the bonding boundary. Therefore, for lithium disilicate, it is recommended that the sandblasting time in clinical practice does not exceed 30 s, and the pressure does not exceed 0.5 MPa.
In addition to traditional physical roughening methods such as sandblasting and emery grinding, many studies in recent years have proposed using lasers for surface treatment. The currently studied lasers mainly include Er:YAG (erbium, yttrium, aluminum, garnet) laser and carbon dioxide laser. Some scholars’ research shows that compared with other lasers such as Nd:YAG and CO₂, the Er:YAG laser does not damage the ceramic surface structure when increasing the surface roughness of the ceramic. However, since laser treatment involves many parameters such as laser type and pulse energy, there is currently no relatively unified conclusion in related research. Further research is still needed on how to coordinate the use of various parameters to obtain better bonding performance.
II. Chemical Modification
The two surface treatments mentioned above are both physical surface modifications to improve the bonding strength. The two methods introduced next are to change the bonding interface of lithium disilicate through chemical treatment.
As we all know, in the acid – etching step of direct resin restoration, we often use phosphoric acid to etch the tooth surface. For lithium disilicate, its most significant feature is that its main component is silicate. Therefore, hydrofluoric acid has the best etching effect. Hydrofluoric acid (HF) reacts with the glass phase in lithium disilicate, that is, SiO₂, to form soluble hexafluorosilicate, and expose needle – like lithium disilicate crystals. The crystals interlock with each other to form a network structure, and widely distributed pores are formed at the connection points, which is conducive to the penetration of the adhesive. In addition, the concept of contact angle needs to be mentioned here. It refers to the angle formed when a liquid contacts a solid surface. The ceramic surface after hydrofluoric acid etching has a low contact angle, forming a highly hydrophilic high – energy surface. Therefore, it is very easy to adsorb moisture, and this stage is very likely to be contaminated.

In addition, silanization is also a commonly used and efficient chemical treatment method. Since the ceramic surface exposes hydroxyl functional groups (silanol groups) after acid – etching, it is easy to react with the hydroxyl groups in the silane coupling agent to form covalent bonds. Therefore, acid – etching – silanization is used as a standard process for surface treatment in clinical practice.
Here we can see that the essence of the silanization reaction is a hydrolysis – condensation reaction, and the condensation reaction releases water molecules. The reaction rate of this reaction is limited by the rate of water molecule release. Therefore, the reaction rate can be accelerated by hot – air drying or extending the water evaporation time. This is why some doctors in clinical practice use a hot air blower to heat the restoration after treatment.

In addition, we need to know that the silane condensation reaction is a surface – limited chemical reaction, that is, the hydrolyzed silane only reacts with the ceramic surface layer, and other hydrolyzed silane layers do not bind to the ceramic surface. Instead, they entangle with the lower layer, and a large number of such loose structures will reduce the bonding strength. Therefore, in clinical applications, it is recommended to apply the silane primer only once, and try to remove the excess silane and the water generated in the condensation reaction.
There have been many previous studies on the acid – etching and silanization treatment of lithium disilicate, including tests and comparisons of the final bonding strength under different reaction conditions such as single use and combined use. Most of the conclusions show that hydrofluoric acid etching is an essential step, and the improvement of bonding strength by acid – etching alone is stronger than that of sandblasting or silanization. However, the optimal hydrofluoric acid concentration + treatment time and silanization product combination for clinical use need to be determined according to the situation, and there is no unified standard. There are certain differences between different brands. It is sufficient to meet the clinical requirements according to the instruction manual.
In addition, in recent years, many bonding products have added silane components to universal adhesives in an attempt to simplify the bonding steps. However, since silane is unstable in acidic solutions, the silanization components in existing universal adhesives do not perform particularly well, and the separate silanization step is still more recommended. But the latest laboratory research has also proposed using new technologies to add silane components to universal adhesives, achieving good bonding strength. In the future, long – term clinical observation may be needed to test whether such new technologies are feasible.
III. Clinical Precautions
Since the physical roughening of restorations such as sandblasting, grinding, and laser treatment in clinical practice is completed by the dental technician, as clinicians, what they need to master is acid – etching and silanization. First, as mentioned before about the contact angle problem, the ceramic surface after sandblasting and hydrofluoric acid etching will form a high – energy hydrophilic surface, which is very easy to be contaminated. The silane coupling agent can convert the high – energy hydrophilic surface into a low – energy hydrophobic surface, thereby reducing the risk of contamination.
Research shows that the most critical step in ceramic bonding is to perform silanization immediately after acid – etching. The surface after silanization can remain stable for a long time, which is conducive to try – in and cleaning to remove excess silane and pollutants.
In actual operation, there are many sources of contamination, including glove talcum powder, try – in paste, saliva, etc. More than one research result shows that the impurities on the surface of lithium disilicate contaminated by saliva cannot be removed only by rinsing with distilled water. This corresponds to the situation in clinical operation where the restoration is contaminated during the process of timely silanization after hydrofluoric acid etching. The conclusion shows that for the surface of lithium disilicate contaminated by saliva, hydrofluoric acid and phosphoric acid etching are effective ways to remove contamination. In addition, in this study, the test results show that the bonding strength of the GB/AB group is stronger than that of SU (universal adhesive), indicating that the bonding effect of the step including silanization primer alone is better. This also shows that the silanization components added in SU may not be effective in bonding.
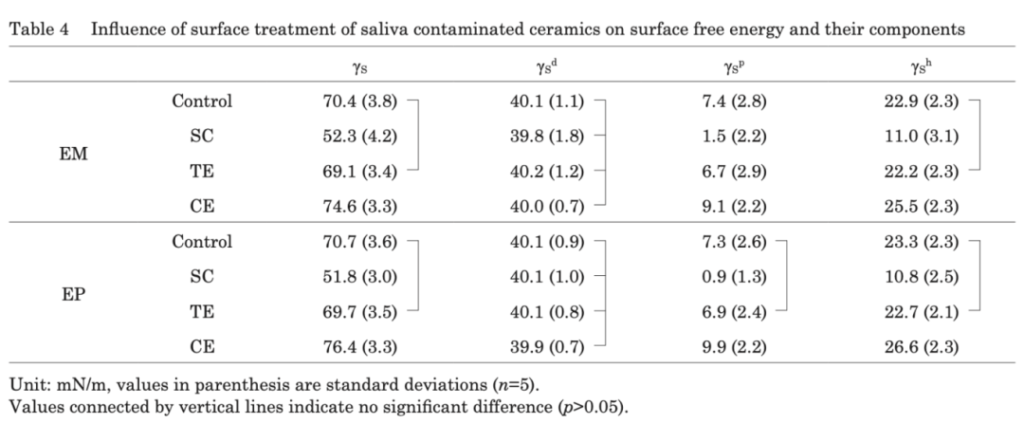
In addition, for the inner surface of the restoration contaminated by saliva, the best cleaning method is to re – etch with HF. However, due to the high toxicity of HF and its non – permission for direct clinical use in some countries, other alternative cleaning methods have also been studied, mainly including: re – silanization, phosphoric acid cleaning, sodium hypochlorite cleaning, and commercial cleaners. The existing research results show that except that simply flushing with a water gun cannot meet the clinical bonding requirements, there is no significant difference between the other several methods and the control group that is directly silanized without contamination, and they can all be used as clinical choices.

For the saliva contamination after hydrofluoric acid etching and immediate silanization, this situation is the closest to our clinical operation process. The research results show that cleaning with ethanol can achieve a bonding strength close to that of the uncontaminated surface; but this study also shows that even if silanization is carried out in a timely manner, once contaminated, it is not enough to clean the surface only with a water gun. In addition, the test also pointed out that re – silanization on the cleaned surface is beneficial to increasing the bonding strength.
Summary
This article mainly introduces four surface treatment methods before bonding starting from the characteristics of the lithium disilicate material itself, and focuses on analyzing the possible detailed treatment problems in the acid – etching and silanization steps in clinical operation. The core issue to understand is: in order to achieve better bonding strength, avoiding contamination is a very important step. Therefore, we should perform moisture isolation before bonding and carry out silanization immediately after acid – etching. If contamination occurs accidentally during the bonding process, we should also know the corresponding treatment methods. If it is contaminated before silanization, the best way should be to re – etch with hydrofluoric acid, or clean it with the corresponding acid – base or special cleaner; if it is contaminated after silanization, only ethanol disinfection and cleaning are needed.
References
[1] Strasser Thomas,Preis Verena,Behr Michael et al. Roughness, surface energy, and superficial damages of CAD/CAM materials after surface treatment.[J].Clin Oral Investig, 2018, 22: 2787 – 2797.
[2] Hou Yepo, Shen Renze, Chen Luyuan, Chen Yi, Jiang Yingtong, Li Jingmei, Gao Jie. Influence of Er:YAG laser irradiation on the bonding performance of machinable lithium disilicate – reinforced lithium disilicate. Journal of Oral Diseases Prevention and Treatment. 2018, 26(2): 95 – 98
[3] Yoshida Fumi,Tsujimoto Akimasa,Ishii Ryo et al. Influence of surface treatment of contaminated lithium disilicate and leucite glass ceramics on surface free energy and bond strength of universal adhesives.[J].Dent Mater J, 2015, 34: 855 – 62.
[4] Byoung I. Suh, Principles of Adhesion Dentistry – A Theoretical and Clinical Guide for Dentists.[M].2013
[5] Lapinska Barbara,Rogowski Jacek,Nowak Joanna et al. Effect of Surface Cleaning Regimen on Glass Ceramic Bond Strength.[J].Molecules, 2019, 24: undefined.
[6] Yoshida Keiichi, Influence of cleaning methods on the bond strength of resin cement to saliva – contaminated lithium disilicate ceramic.[J].Clin Oral Investig, 2020, 24: 2091 – 2097.




Leave a Reply