Human Amniotic Membrane as Therapeutic Agent in Pulpotomy of Permanent Molars
For many years, the treatment of reversible and irreversible inflamed dental pulp has been controversial. For the treatment of reversible inflamed dental pulp, vital pulp therapy is widely accepted under a high degree of consensus. However, even with years of progress in the field of conservative dentistry, for irreversible inflamed dental pulp, the most accepted treatment method is still root canal treatment (RCT). Before root canal treatment, pulp amputation is usually used as an emergency treatment method for exposed pulp of mature permanent teeth. However, the opposing view holds that the injured dental pulp should not always be completely removed. The best root canal filling material is healthy dental pulp tissue. A meta-analysis reported the long-term success rate of pulp amputation for permanent teeth with irreversible pulpitis in the existing literature. This challenges the statement that pulp amputation is contraindicated in teeth with irreversible pulpitis and the view that root canal treatment is the only option. Pulp amputation is a kind of vital pulp therapy for exposed dental pulp, aiming to maintain its vitality, function and keep it asymptomatic. This procedure is based on the concept of surgically removing the presumably inflamed coronal pulp while protecting the remaining radicular pulp at the root canal orifice with suitable materials. Such materials protect the dental pulp, promote healing and prevent further damage. Complete coronal pulp amputation has a high success rate in treating vital pulp exposure of carious permanent teeth with closed apices. Many materials have been used as pulp amputation agents, among which the oldest and once regarded as the gold standard is formalin. However, due to its controversial properties, formalin remains the most controversial material used in dentistry to date. Other materials such as mineral trioxide aggregate (MTA), ferric sulfate, calcium hydroxide, MTA plus platelet-rich fibrin (PRF), MTA plus triple antibiotic paste, etc. have also been widely studied as pulp amputation agents. However, due to their synthetic sources, all these materials are accompanied by complications and adverse effects on teeth. Therefore, biologically derived natural pulp amputation agents may provide a better alternative for the treatment of permanent teeth with irreversible pulpitis.
The human amniotic membrane (AM) is located on the inner side of the fetal placenta and defines the fluid-filled amniotic cavity. It consists of three histological structures: the epithelial layer, the basement membrane and the avascular stromal layer. Although the AM is avascular, it has multiple metabolic functions and is responsible for the transport of water and soluble substances, and the production of bioactive factors such as growth factors, cytokines and vasoactive peptides. The AM extracted from discarded placentas has been used in the medical profession for a long time. Davis et al. first reported its application in skin grafts in 1910. Sabella was the first to present clinical cases of using AM for burn management. Since then, numerous uses of AM have been discovered in fields such as ophthalmology, otolaryngology (ENT), plastic surgery and dentistry. The application of AM in root canal treatment is relatively new, so there is not much relevant literature. There have been reports of successful cases of using AM for regenerative root canal treatment in non-vital immature teeth. The literature also reported the use of AM as a barrier membrane to manage periodontal lesions with poor prognosis. The application of AM in dental surgery benefits from its various beneficial properties. AM has anti-inflammatory, antibacterial, antiviral and anti-tumor properties, and can also relieve pain when applied. The pluripotency of amniotic membrane-derived cells and their ability to form scaffolds contribute to regeneration. In addition, it involves fewer ethical issues, making it a very convenient material. Human AM is an “immunologically privileged” allograft because it lacks immune markers. It comes from placentas that have been freeze-dried under vacuum at -60 °C for 48 hours. For sterilization, a radiation of 2.5 megarads is used in a batch-type cobalt-60 irradiator. This membrane is easy to obtain, has a long shelf life and simple application techniques.
The application of AM in various periodontal and maxillofacial surgeries has been widely studied. However, there are few remaining studies on its use in the field of root canal treatment. So far, there has been no research on using AM as a pulp amputation agent for permanent teeth. Therefore, this case report aims to utilize the beneficial properties of human AM and use it as a pulp amputation agent to manage carious permanent teeth with irreversible pulpitis.
Case Report
The patient was a 22-year-old female with no special past medical history. She complained of pain and sensitivity in the mandible for 1 month. Clinical examination revealed a moderately sized deep carious exposure in her right lower permanent molar (Figure 1A). In the past month, she had a history of mild to moderate pain when consuming cold drinks, and there was no history of any previous pain or swelling. No sinus tract or alveolar bone swelling related to the affected tooth was seen. The percussion pain response was negative. Thermal testing showed persistent pain under heating and cold stimulation. The results of the electric vitality test indicated that the response of the affected tooth was stronger than that of the contralateral healthy mature permanent tooth. Subsequently, a radiographic examination was performed on the affected area, and the results showed a large radiolucent shadow in the crown of the suspected tooth, close to the dental pulp. The periapical area looked healthy, with no periapical radiolucent shadow and good bone structure. The periodontal ligament space and lamina dura appeared normal (Figure 2A). After completing a comprehensive clinical and radiographic examination, we diagnosed the affected tooth with irreversible pulpitis. After obtaining the patient’s consent, a complete coronal pulp amputation using human amniotic membrane (AM) as a therapeutic agent was planned.
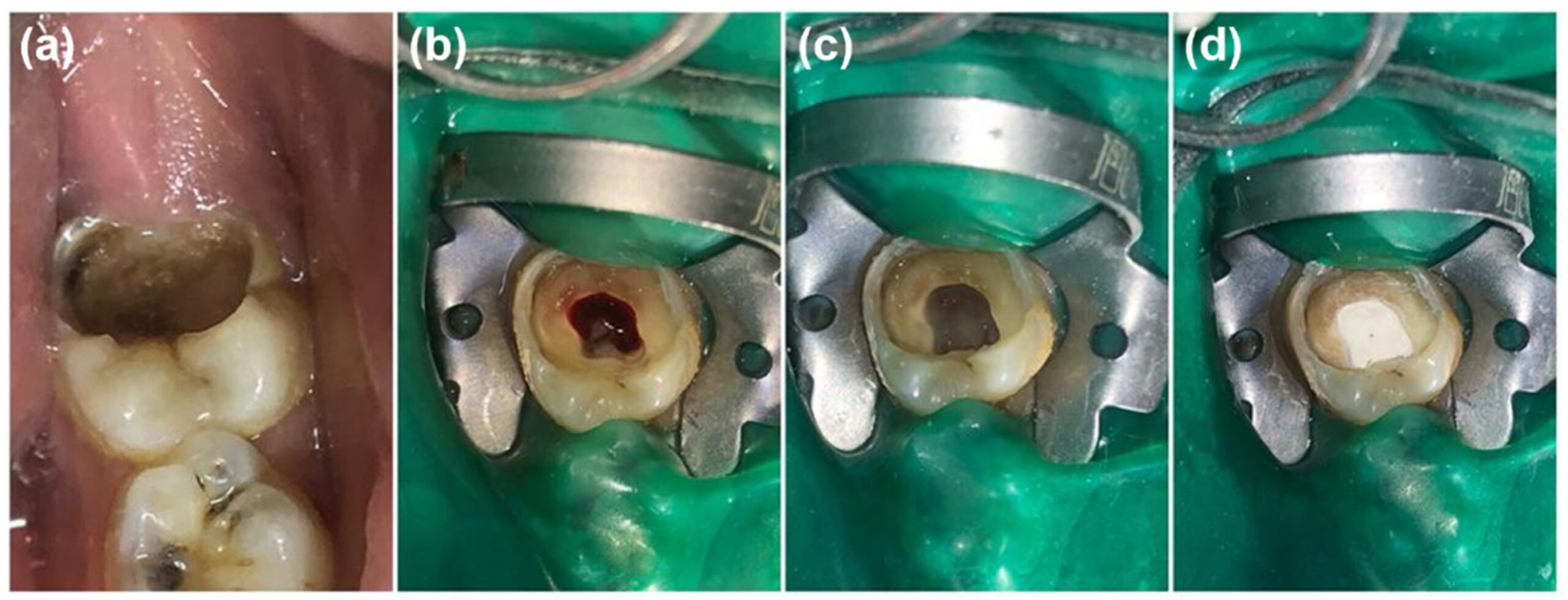
Figure 1
(A) Preoperative clinical photograph of tooth 46. (B) Removal of the carious part, resulting in exposure of the pulp chamber. (C) Hemostasis at the root canal orifice. (D) Clinical view of the human amniotic membrane being cut to the required size. (E) Placement of the amniotic membrane in the tooth cavity.
(F) Postoperative photograph of tooth 46.
Figure 2
(A) Preoperative X-ray of tooth 46. (B) Immediate postoperative X-ray showing glass ionomer cement (GIC) and temporary restoration. (C) Follow-up X-ray one month after surgery. (D) Follow-up X-ray three months after surgery. (E) Follow-up X-ray nine months after surgery.
(F) Follow-up X-ray eighteen months after surgery.
Anesthesia was achieved using 2% lidocaine with 1:80,000 adrenaline. The cavity was prepared using a high-speed handpiece under rubber dam isolation. The coronal pulp tissue was removed with a sterile curette until reaching the root canal orifice. Hemorrhage from the root canal stump was stopped by gently applying a cotton ball soaked in sterile normal saline and maintaining it for 5 minutes. The pulp chamber was rinsed with normal saline to remove any remaining pulp fragments. AM was obtained from the tissue bank of Tata Memorial Hospital in Mumbai, India. The freshly freeze-dried AM was cut into small pieces using autoclaved surgical scissors and activated by soaking in normal saline for 1 minute (Figure 1D). Then it was gently placed at the bottom of the pulp to form a uniform layer (2 – 3 mm) (Figure 1E). Care was taken to cover all root canal orifices and avoid the membrane adhering to the cavity walls. Universal restorative glass ionomer cement (GC) was placed on the membrane, and a temporary filling was made using Cavit-G (3M ESPE) (Figure 1F). Postoperatively, the patient was instructed to avoid eating or drinking within half an hour and avoid chewing on the affected side within 24 hours. If pain occurred, analgesics (acetaminophen) could be taken as needed. One week later, a permanent filling was performed using light-cured composite resin (Brilliant-NG; Coltene). The patient was followed up at 1, 3, 9 and 18 months after surgery and was asked to report any discomfort or symptoms that occurred.
Follow-up
The patient reported mild heat and cold sensitivity by phone one day after the pulpotomy. It gradually subsided in the following week. At the 1, 3, 9 and 18-month follow-ups, the tooth was completely asymptomatic (Figure 2C – F), and the sensitivity test of the tooth was similar to that of the control tooth. Radiographic examination showed that the periodontal ligament space was normal and the trabecular bone morphology was close to normal (Figure 2).
Discussion
Removing the dental pulp from the root canal and filling it with bio-inert materials will inevitably lead to changes in the biomechanical properties of the tooth. Loss of vitality after root canal treatment changes the tooth in three main aspects – composition, dentin structure and tooth macrostructure. These changes have multiple clinical implications, such as increased tooth brittleness, reduced adhesion to the matrix and decreased stability of restorations. In addition, regardless of the doctor’s experience, the complexity of the root canal system always poses a challenge. In view of the above arguments, the risk-benefit ratio of attempting root canal treatment should always be considered. Since the radicular pulp is usually not infected, it is removed to avoid further infection of the root canal system. Therefore, root canal treatment can sometimes be classified as “preventive”. As conservative dentists, the vitality of teeth should be preserved as much as possible.
Çalişkan successfully treated inflamed permanent teeth through pulp amputation. Using various pulp amputation drugs also brings inevitable disadvantages. Some of these drugs are highly controversial, such as formalin. According to Chen et al.’s research, almost all pulp amputation agents have certain adverse effects, such as calcification degeneration, discoloration, external or internal resorption. In this study, we recognized that all the currently used agents are synthetic materials. Therefore, we looked for naturally derived (autograft/allograft) pulp amputation agents.
Although there are few reports on the use of AM in the process of root canal treatment, there have been successful cases. Prasad et al. conducted a study comparing AM and formalin as pulp amputation agents for primary molars. The results showed that AM performed comparably to formalin clinically and radiologically. Suresh et al. reported a case of successful regenerative root canal treatment in a non-vital immature permanent incisor using AM as a novel scaffold. There have also been reports on the use of AM in guided tissue regeneration after periapical root canal surgery. Chi et al. studied the healing of the apical bone after placing AM. It was observed that the healing process was smooth, rapid and extensive after 5 months. Uppada et al. reported the successful combination of AM with hydroxyapatite and platelet-rich fibrin (PRF) in regenerative apical surgery. The aim was to combine the regenerative and wound healing properties of the membrane.
To our knowledge, there has been no case report on using AM as a pulp amputation agent in carious permanent teeth. It is said that AM is comparable to the PRF membrane. PRF has been studied as a pulp amputation agent in combination with MTA and Biodentine. Despite its success, obtaining PRF requires collecting the patient’s blood through venipuncture, which may not be accepted by all patients. The formation of PRF requires special centrifuges and membrane-forming equipment, which makes its operation cumbersome. In this study, no auxiliary materials were used with the amniotic membrane to evaluate its true ability as a pulp amputation agent. The freeze-dried membrane was prepared by soaking in normal saline for 1 minute before application. This activation restored the membrane to its fresh layered structure. AM has anti-inflammatory properties and excellent revascularization properties. Direct application of the activated membrane to the amputated dental pulp may have promoted faster healing and recovery of the remaining radicular pulp. As shown in our study, the dental pulp recovered to a normal, non-inflamed state. The scaffold-forming property of the membrane may have stimulated growth factors, which in turn activated undifferentiated dental pulp stromal cells. AM is easy to obtain, cost-effective, requires no special equipment, and has a simple placement technique. Gamma-ray irradiation reduces the possibility of cross-infection through the membrane. However, one limitation of using AM as a pulp amputation agent comes from its ability to stimulate undifferentiated stromal cells to transform into osteoclasts, which may lead to initial internal resorption, as reported by Prasad et al. However, in the long run, odontoblasts are also stimulated, and this effect, combined with the other healing properties of the amniotic membrane, overcomes the resorption process and enables recovery. However, the chance of encountering resorption is reportedly very rare. Our current case did not show internal or external resorption at any stage. AM is a very fragile membrane, so sometimes unskilled operation may also be a limitation.
In conclusion, AM, as a pulp amputation agent, showed good results in this study. Its advantages include anti-inflammatory properties, promoting healing, being easy to obtain and cost-effective. However, when using it, attention should also be paid to its potential limitations, such as possible initial internal resorption and the fragility of the membrane itself. Future research should further explore the application potential of AM in dental pulp treatment and evaluate its long-term effects and safety.
References
- Harty FJ, Chong BS. Harty’s endodontics in clinical practice. Edinburgh; New York: Churchill Livingstone/Elsevier, 2010.
- Li Y, Sui B, Dahl C, et al.. Pulpotomy for carious pulp exposures in permanent teeth: a systematic review and meta-analysis. J Dent 2019;84:1–8. 10.1016/j.jdent.2019.03.010
- ÇALIŞKAN MK. Pulpotomy of carious vital teeth with periapical involvement. Int Endod J 1995;28:172–6. 10.1111/j.1365-2591.1995.tb00293.x
- Alqaderi H, Lee C-T, Borzangy S, et al.. Coronal pulpotomy for cariously exposed permanent posterior teeth with closed apices: a systematic review and meta-analysis. J Dent 2016;44:1–7. 10.1016/j.jdent.2015.12.005
- Holan G, Fuks AB, Ketlz N. Success rate of formocresol pulpotomy in primary molars restored with stainless steel crown vs amalga. Pediatr Dent 2002;24:212–6.
- Kim S, Liu M, Simchon S, et al.. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc 1992;88 Suppl 1:387–92.
- Smaïl-Faugeron V, Glenny A-M, Courson F, et al.. Pulp treatment for extensive decay in primary teeth. Cochrane Database Syst Rev 2018;5:CD003220. 10.1002/14651858.CD003220.pub3
- Erdem AP, Guven Y, Balli B, et al.. Success rates of mineral trioxide aggregate, ferric sulfate, and formocresol pulpotomies: a 24-month study. Pediatr Dent 2011;33:165–70.
- Chen Y, Chen X, Zhang Y, et al.. Materials for pulpotomy in immature permanent teeth: a systematic review and meta-analysis. BMC Oral Health 2019;19:1–9. 10.1186/s12903-019-0917-z
- Mamede AC, Carvalho MJ, Abrantes AM, et al.. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 2012;349:447–58. 10.1007/s00441-012-1424-6
- Toda A, Okabe M, Yoshida T, et al.. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 2007;105:215–28. 10.1254/jphs.CR0070034
- Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol 1960;79:1070–3. 10.1016/0002-9378(60)90512-3
- Benirschke K, Burton GJ, Baergen RN. Maternal diseases complicating pregnancy: diabetes, tumors, preeclampsia, lupus anticoagulant. In: Pathology of the human placenta. Berlin, Heidelberg: Springer, 2012: 495–555.
- Cunningham F, Leveno K, Bloom S, eds. Williams obstetrics. 24 edn. Mcgraw-hill, 2014.
- Davis JS. Ii. skin grafting at the johns hopkins hospital. Ann Surg 1909;50:542–9. 10.1097/00000658-190909000-00002
- Sabella N. Use of the fetal membranes in skin grafting. Medication Reconciliation 1913;83:478–80 https://scirp.org/reference/referencespapers.aspx?referenceid=1209473
- Joseph EJ, Karuna MY, Rao A, et al.. A novel regenerative endodontic procedure in a traumatized immature tooth using amniotic membrane. Dent Res J 2021;18:28.
- Suresh N, Arul B, Kowsky D, et al.. Successful regenerative endodontic procedure of a nonvital immature permanent central incisor using amniotic membrane as a novel scaffold. Dent J 2018;6:36. 10.3390/dj6030036
- Elangovan R, Theyagarajan R. Amniotic membrane as barrier membrane in endo-perio lesion – a interdisciplinary approach. J Dent Oral Health 2019;1:1 http://www.jscholaronline.org/full-text/JDOH/6_401/Amniotic-Membrane.php
- Kumar KA, Chakravarthy M, Selvarajan S, et al.. Use of an amniotic membrane as a novel barrier in a tooth with a questionable prognosis. J Indian Soc Periodontol 2017;21:237. 10.4103/jisp.jisp_67_16
- Chopra A, Thomas BS. Amniotic membrane: a novel material for regeneration and repair. J Biomim Biomater Tissue Eng 2013;18:106. 10.4172/1662-100X.1000106
- Bassir MM, Labibzadeh A, Mollaverdi F. The effect of amount of lost tooth structure and restorative technique on fracture resistance of endodontically treated premolars. J Conserv Dent 2013;16:413–7. 10.4103/0972-0707.117494
- Hargreaves KM, Cohen S, Berman LH. Cohen’s pathways of the pulp. 10 edn. St. Louis, Mo: Mosby Elsevier, 2011: 952.
- Calişkan MK. Pulpotomy of carious vital teeth with periapical involvement. Int Endod J 1995;28:172–6. 10.1111/j.1365-2591.1995.tb00293.x
- Prasad MG, Adiya PV, Babu DN. Amniotic membrane versus formocresol as pulpotomy agents in human primary molars: an in vivo study. Pesquisa Brasileira em Odontopediatria e Clinica Integrada 2017;17:26. 10.4034/PBOCI.2017.171.50
- Chi CS, Andrade DB, Kim SG, et al.. Guided tissue regeneration in endodontic surgery by using a bioactive resorbable membrane. J Endod 2015;41:559–62. 10.1016/j.joen.2014.10.018
- Uppada UK, Kalakonda B, Koppolu P, et al.. Combination of hydroxyapatite, platelet rich fibrin and amnion membrane as a novel therapeutic option in regenerative periapical endodontic surgery: case series. Int J Surg Case Rep 2017;37:139–44. 10.1016/j.ijscr.2017.06.009
- Kumar V, Juneja R, Duhan J, et al.. Comparative evaluation of platelet-rich fibrin, mineral trioxide aggregate, and calcium hydroxide as pulpotomy agents in permanent molars with irreversible pulpitis: a randomized controlled trial. Contemp Clin Dent 2016;7:512. 10.4103/0976-237X.194107
- Keswani D, Pandey RK, Ansari A, et al.. Comparative evaluation of platelet-rich fibrin and mineral trioxide aggregate as pulpotomy agents in permanent teeth with incomplete root development: a randomized controlled trial. J Endod 2014;40:599–605. 10.1016/j.joen.2014.01.009
- D. Prasanthi NV, Simpsy G, Chittem J, et al.. Biological approach in the management of permanent molars with irreversible pulpitis using platelet-rich fibrin as a pulpotomy medicament: case reports with 2-year follow up. J Interdiscip Dentistry 2018;8:30. 10.4103/jid.jid_54_17
- Hiremath H, Saikalyan S, Kulkarni SS, et al.. Second-generation platelet concentrate (Prf) as a pulpotomy medicament in a permanent molar with pulpitis: a case report. Int Endod J 2012;45:105–12. 10.1111/j.1365-2591.2011.01973.x
- Naik B, Karunakar P, Jayadev M, et al.. Role of platelet rich fibrin in wound healing: a critical review. J Conserv Dent 2013;16:284. 10.4103/0972-0707.114344
- Kobayashi M, Kawase T, Horimizu M, et al.. A proposed protocol for the standardized preparation of prf membranes for clinical use. Biologicals 2012;40:323–9. 10.1016/j.biologicals.2012.07.004
- Kothari CR, Goudar G, Hallur N, et al.. Use of amnion as a graft material in vestibuloplasty: a clinical study. Br J Oral Maxillofac Surg 2012;50:545–9. 10.1016/j.bjoms.2011.09.022
- Koob TJ, Lim JJ, Massee M, et al.. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–62. 10.1002/jbm.b.33141
Author:Saumya Johri, Promila Verma, Rhythm Bains, Aseem Praksh Tikku




Leave a Reply