Due to the biocompatibility of titanium, it is generally considered that titanium does not cause allergic reactions. Although there are very few clinical reports regarding titanium-induced contact dermatitis (CD) or granulomatous reactions in dental implants, cardiac pacemakers, hip prostheses, surgical clips, and internal fixation of fractures, titanium allergy remains a topic of ongoing debate. Titanium allergy is characterized by localized aggregates of macrophages and T lymphocytes, along with a lack of B lymphocytes, thus pointing to a Type IV hypersensitivity reaction. The occurrence of hypersensitivity documented in a small number of patients raises the question of whether certain patients may exhibit metal sensitivity after exposure to titanium under specific circumstances. With advancements in smelting technology, the use of titanium has increased, leading to greater possibilities of human allergy to this metal. This case report illustrates the emergence of allergic symptoms one week after the placement of a dental implant. Although there were no oral or facial signs after the implant was placed, eczema symptoms were observed in distal body parts, including the hands, skin, and back. The symptoms completely disappeared after the dental implant was removed.
Case Report
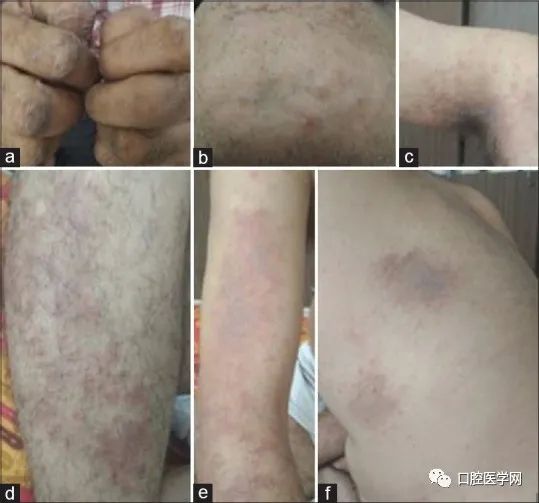
A 56-year-old male patient, who denied any allergy history, visited the periodontal department requesting the removal of a well-placed implant with a restoration at the site of the upper left central incisor. The patient’s medical history indicated that he underwent dental implant surgery in the upper left central incisor area at a private dentist six months prior. Within a week post-surgery, the patient noticed widespread rashes on nearly all body surfaces, including the armpits, groin, chest, shoulders, forearms, and hands (Figure 1), without any significant oral signs or symptoms.

Preoperative appearance of the patient, showing rashes and erythema on (a) fingers, (b) chin, (c) armpits, (d) legs, (e) arms, and (f) back.
After this, he first consulted a dermatologist 10 days after the implant placement, who believed it to be a skin issue and diagnosed it as generalized pruritus. Two weeks after the implant, hematological reports showed elevated eosinophil counts. The doctor prescribed antihistamines (H1 antagonists) as an anti-allergy medication, but the eczema did not improve. As there was no significant improvement after the antihistamine treatment, the dermatologist suggested a skin biopsy two months later to rule out herpetic dermatitis and gliadin antibodies (IgG, IgA), as well as a tissue transglutaminase (Ttg) test to exclude celiac disease. All test results were negative. No significant pathological changes were observed around the implant (Figure 2), and imaging showed normal surrounding bone tissue. The patient was in good overall health, without any drug or other relevant history. He visited multiple dermatology clinics and hospitals and took antihistamines, topical, and systemic steroids, only experiencing brief symptom relief, with rashes reappearing after discontinuation.

Surgical removal of the implant: (a) preoperative X-ray, (b) full-thickness flap reflection and visualization of the surgical area, (c) extracted implant, (d) extracted implant, abutment, and screw-retained restoration, (e) flap closure and suturing, (f) postoperative X-ray.
Four months later, the patient returned to the oral surgery department requesting the removal of the implant. Considering there were no significant symptoms around the oral area, the dentist advised him against removal and placed a dental crown instead. After regular medication and consultations with the dentist and dermatologist, he ultimately came to the periodontal department requesting the removal of the implant. Based on comprehensive clinical symptoms, laboratory tests, and opinions from dermatology and internal medicine, titanium implant allergy was diagnosed, and the patient was advised to undergo patch testing before the implant removal. The patient refused patch testing, and upon obtaining consent, the implant was removed, and clinical images of the patient were taken. Due to a significant alleviation of symptoms, antihistamines were discontinued after one week. For aesthetic reasons, the patient accepted removable partial denture restoration. At a six-month follow-up, all cutaneous lesions had completely resolved, with no signs of itching or pain (Figure 3). The patient showed significant improvement, with symptoms fully alleviated.
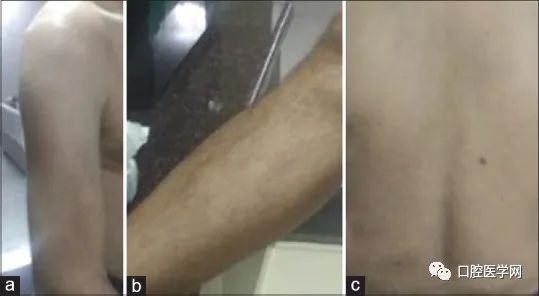
At the six-month follow-up, the patient showed successful symptom relief: (a) arm, (b) leg, and (c) back.
Discussion
Titanium and its alloys are used as biocompatible materials for various applications, such as artificial joints and pacemakers, and have been widely used in the manufacture of dental implants over the past 20-30 years. Dental implants are made from commercially pure titanium (CPTi) and titanium alloys (usually CPTi Grade 4). However, the biocompatibility of any material cannot be considered universal. It has been reported that the probability of developing metal sensitivity after contact with titanium implants is approximately 0.6%. Although titanium is widely used for alloy manufacturing due to its biocompatibility, it remains one of the irritating allergens due to nickel leaching during the production process. Different titanium manufacturers have been found to contain up to 0.034 wt% nickel even in standard titanium alloys (TiAl6Nb7 and TiAl6V4). However, its presence remains controversial due to inconsistent conclusions from allergy testing and inadequate preparation for patch testing. A literature review by Kim et al. reported that the number of titanium elements in the human body increases due to internal exposure after implantation, thereby raising the concentration of titanium ions in surrounding tissues, regional lymph nodes, and lung tissue. Titanium implants have been shown to stimulate Type I or IV hypersensitivity reactions due to the release of immunogenic protein-metal complexes. Hypersensitivity reactions are also associated with various issues, such as atopic dermatitis and impaired fracture healing, pain, and necrosis. There are documented cases in oral implantology of facial erythema, edema, and proliferative tissue appearance. Preez et al. reported a case of severe local tissue reaction that required the removal of the implant in 2007. In 2008, Egusa et al. reported cases of eczema associated with titanium dental implants. According to the criteria set by Albrektsson et al., such cases should be classified as biological failure and included in discussions of early implant failure. CD is a locally delayed-type hypersensitivity reaction induced by various chemicals and metals. In the dental field, implant allergies are often overlooked. Allergy diagnostic tests include patch testing, but to date, there is no standardized titanium patch test. Memory lymphocyte immune stimulation tests have also been developed but lack specificity for detecting lymphocyte proliferation. Lymphocyte transformation tests have been reported to provide many false-positive results. With the rapid increase in the use of titanium plates and dental implants in dentistry, the incidence of titanium sensitivity is rising. Before undergoing implant surgery, it is advisable to assess any previous history of allergy to metals or jewelry, and ideally, recommend metal allergy testing. Implantologists should be aware of the potential for titanium allergy. When evaluating the allergic response to titanium, there are many limitations in diagnostic uncertainties, and in most cases, it is considered a rare occurrence, a finding summarized in the literature review by Goutam et al. However, Basketter et al. and Okamura et al. suggested the usefulness of testing with other titanium salts in cases of suspected titanium allergy.
Conclusion
This clinical report presents a suspected allergic reaction induced by titanium dental implants, which is often overlooked despite the need for interrelated considerations. Therefore, further discussion and investigation of the rare occurrences of allergic reactions to titanium materials in clinical dentistry are warranted.
References
1. Abdallah HI, Balsara RK, O’Riordan AC. Pacemaker contact sensitivity: Clinical recognition and management. Ann Thorac Surg. 1994;57:1017–8.
2. Verbov J. Pacemaker contact sensitivity. Contact Dermatitis. 1985;12:173.
3. Viraben R, Boulinguez S, Alba C. Granulomatous dermatitis after implantation of a titanium-containing pacemaker. Contact Dermatitis. 1995;33:437.
4. Yamauchi R, Morita A, Tsuji T. Pacemaker dermatitis from titanium. Contact Dermatitis. 2000;42:52–3.
5. Lalor PA, Gray AB, Wright S, Railton GT, Freeman MA, Revell PA. Contact sensitivity to titanium in a hip prosthesis? Contact Dermatitis. 1990;23:193–4.
6. Lalor PA, Revell PA, Gray AB, Wright S, Railton GT, Freeman MA. Sensitivity to titanium? A cause of implant failure. J Bone Joint Surg Br. 1991;73:25–8.
7. Witt JD, Swann M. Metal wear and tissue response in failed titanium alloy total hip replacements. J Bone Joint Surg Br. 1991;73:559–63.
8. Hunt J, Williams D, Ungersbock A, Perrin S. The effect of titanium debris on soft tissue response. J Mater Sci Mater Med. 1994;5:381–3.
9. Sicilia A, Cuesta S, Coma G, Arregui I, Guisasola C, Ruiz E, et al. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008;19:823–35.
10. Peters MS, Schroeter AL, van Hale HM, Broadbent JC. Pacemaker contact sensitivity. Contact Dermatitis. 1984;11:214–8.
11. Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5:10.
12. Onodera K, Ooya KO, Kawamura H. Titanium lymph node pigmentation in the reconstruction plate system of a mandibular defect. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1993;75:495–7.
13. Parr GR, Gardner LK, Toth RW. Titanium: The mystery metal of implant dentistry. Dental materials aspects. J Prosthet Dent. 1985;54:410–4.
14. Schedle A, Ortengren U, Eidler N, Gabauer M, Hensten A. Do adverse effects of dental materials exist? What are the consequences, and how can they be diagnosed and treated. Clin Oral Implants Res. 2007;18:232–56.
15. Thomas P, Bandl WD, Maier S, Summer B, Przybilla B. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: Case report and review of the literature. Contact Dermatitis. 2006;55:199–202.
16. Bircher AJ, Stern WB. Allergic contact dermatitis from “titanium” spectacle frames. Contact Dermatitis. 2001;45:244–5.
17. Preez LA, Butow KW, Swart TJ. Implant failure due to titanium hypersensitivity/allergy? Report of a case. SADJ. 2007;62:24–5.
18. Egusa H, Ko N, Shimazu T, Yatani H. Suspected association of an allergic reaction with titanium dental implants: A clinical report. J Prosthet Dent. 2008;100:344–7.
19. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25.
20. Valentine-Thon E, Schiwara HW. Validity of MELISA for metal sensitivity testing. Neuro Endocrinol Lett. 2003;24:57–64.
21. Goutam M, Giriyapura C, Mishra SK, Gupta S. Titanium allergy: A literature review. Indian J Dermatol. 2014;59:630.
22. Basketter DA, Whittle E, Monk B. Possible allergy to complex titanium salt. Contact Dermatitis. 2000;42:310–1.
23. Okamura T, Morimoto M, Fukushima D, Yamane G. A skin patch test for the diagnosis of titanium allergy. J Dent Res. 1999;78:1135.




Leave a Reply